About Us
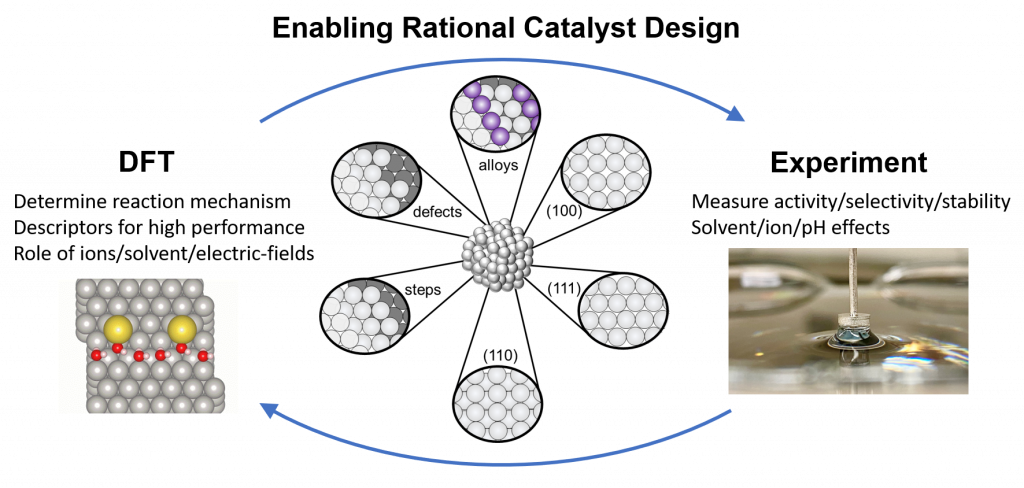
The McCrum Group utilizes a combination of computational modeling and detailed experiments on well-defined materials to understand catalysis, electrochemistry, and surface science at the atomic scale.
This fundamental insight allows us to intellegently design more active, selective, and stable low-cost catalysts and processes for efficient energy conversion and storage, water treatment, and sustainable chemical production.
Research Interests
Sustainable Chemical Production
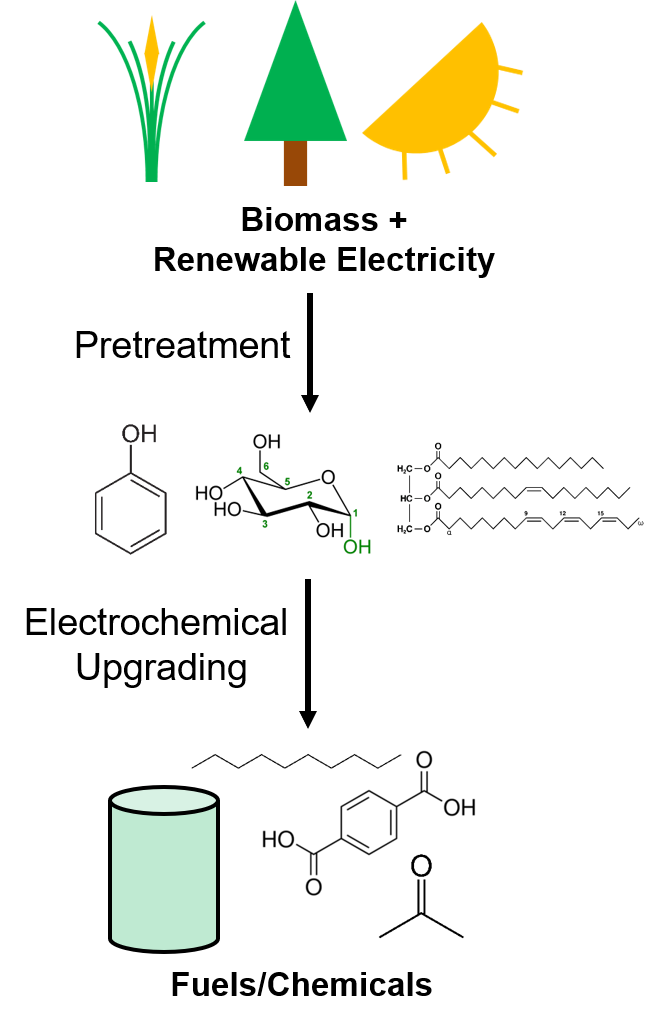
Reducing the carbon footprint of fuels and chemicals requires the development of sustainable routes of production. Electrocatalysis is a powerful tool that can use renewable electricity to enable sustainable fuel and chemical production.
This could include converting biomass or CO2 into solvents and precursors for plastics, to close the carbon cycle.
Water can be split with renewable electricity to produce sustainable hydrogen – to be used as an industrial feedstock.
Nitrogen from the atmosphere can be converted to sustainable fertilizers.
Electrode/Electrolyte
Interface Design
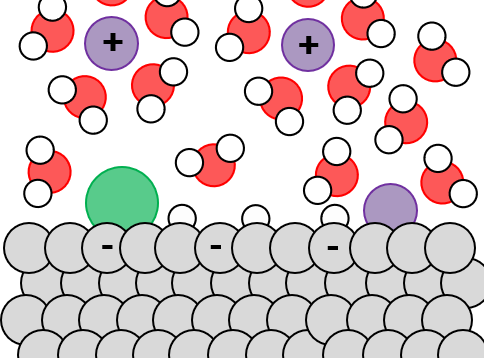
The electrode/electrolyte interface is complex. The presence of solvent, ions, and electric fields near to the (electro)catalyst surface drive large changes in reaction activity (the rate of the reaction), reaction selectivity (ratio of desired product to undesired byproducts), and catalyst stability.
Using a combination of computational modeling and detailed experiments, we understand the mechanism of these changes at the atomic scale — allowing us to predictively design improved electrolytes.
Alloy Catalyst Design
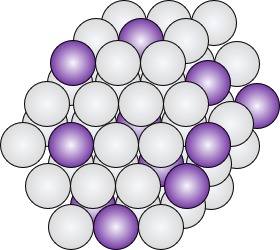
While the behavior of pure metal catalysts are fairly well understood, the design and synthesis of alloy catalysts, which offer a broader array of properties, is complicated and typically left to trial and error.
By understanding the behavior of complex alloys at the atomic scale, we can develop techniques to intelligently and predictively design advanced, high-performance, low-cost alloy catalysts.